FDA Clears Gel-Based Device That Instantly Stops Severe Bleeding: What To Know About Traumagel
Topline
Traumagel—the first gel-based therapy to prevent life-threatening bleeding—was cleared for market use Thursday, and its manufacturer said its fast-acting properties and the eliminated need for direct contact set it apart from its competitors.
Cresilon receives FDA 510(k) clearance for TRAUMAGEL
Key Facts
Drug manufacturer Cresilon announced Thursday the Food and Drug Administration granted 510(k) clearance—the ability to market a product—to its medical device Traumagel.
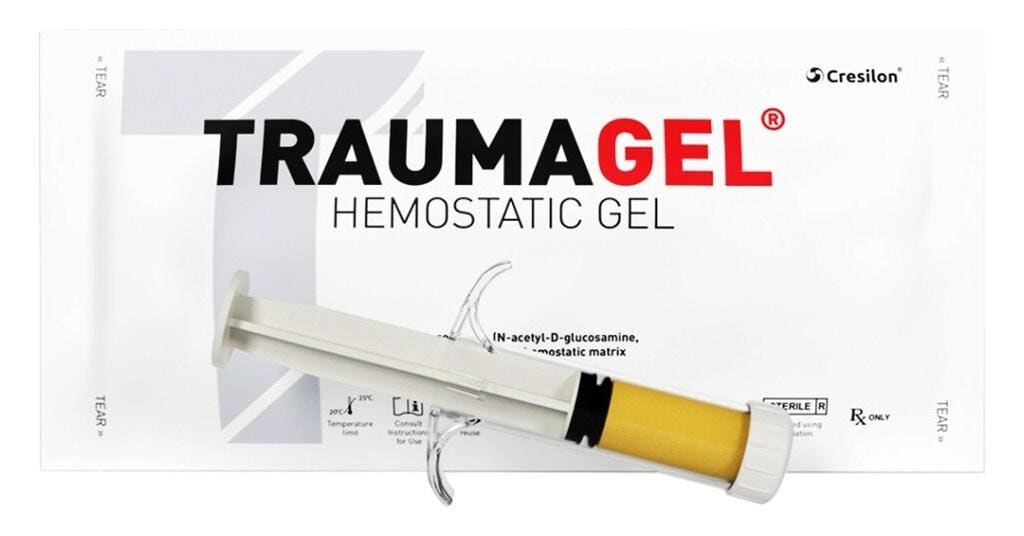
Traumagel is an algae-derived gel that comes in pre-filled syringes, and is applied to traumatic wounds and severe injuries—like gunshot wounds and stab wounds—to stop and control severe blood loss.
The product—which will be available in 2024—will be developed as a quick and effective solution to life-threatening bleeding for emergency medical services systems, the U.S. military, government health agencies and other medical professionals.
Traumagel is different from currently available treatment methods like gauze and tourniquets because the gel doesn’t require pressure to work, and its effects happen within seconds, unlike other methods that can take several minutes to kick in, Joe Landolina, CEO of Cresilon, told Forbes.
Though he didn’t give a specific number, Landolina said Traumagel will be priced “competitively” to other treatments on the market, adding the product has to be accessible “in order to save lives.”
How Does Traumagel Work?
Traumagel is the first gel-based hemostatic agent—treatments used to stop bleeding—cleared for use in the U.S. It’s a hummus-like gel that’s inserted into a bleeding wound via a syringe. Once it’s inserted, it allows the patient to form their own blood clots and stop the bleeding. It eliminates the need to pack wounds, and it’s able to get into the “nooks and crannies,” allowing for much faster results, according to Landolina. It’s also safer for the medical professional who’s applying it because unlike other treatments, it doesn’t require direct contact with the wound or the patient’s blood, and prevents potential injury from bone or bullet fragments, Landolina said.
What Is The Difference Between Fda Clearance And Approval?
The FDA classifies medical devices into three categories: Class I, Class II and Class III. Class I and Class II devices must file a 510(k) submission—the process for medium-risk devices—to prove the product is safe before hitting the market. FDA clearance means the manufacturer has demonstrated the product is as safe and effective as an already legally marketed device. Class III devices are considered higher risk, so they have to go through the agency’s most rigorous process, which involves getting premarket approval. FDA approval means the product’s benefits outweigh the known risks.
Big Number
40%. That’s how many global trauma-related deaths are caused by bleeding, according to the Department of Homeland Security. It’s estimated around 1.5 million people worldwide die from hemorrhage every year.
Tangent
Cresilon also had another product cleared by the FDA last summer that’s based on the same technology as Traumagel. That device—called Cresilon Hemostatic Gel—is meant to stop bleeding from minor cuts and scrapes in humans.